What is a Solvent? — Definition & Examples
Solvent definition
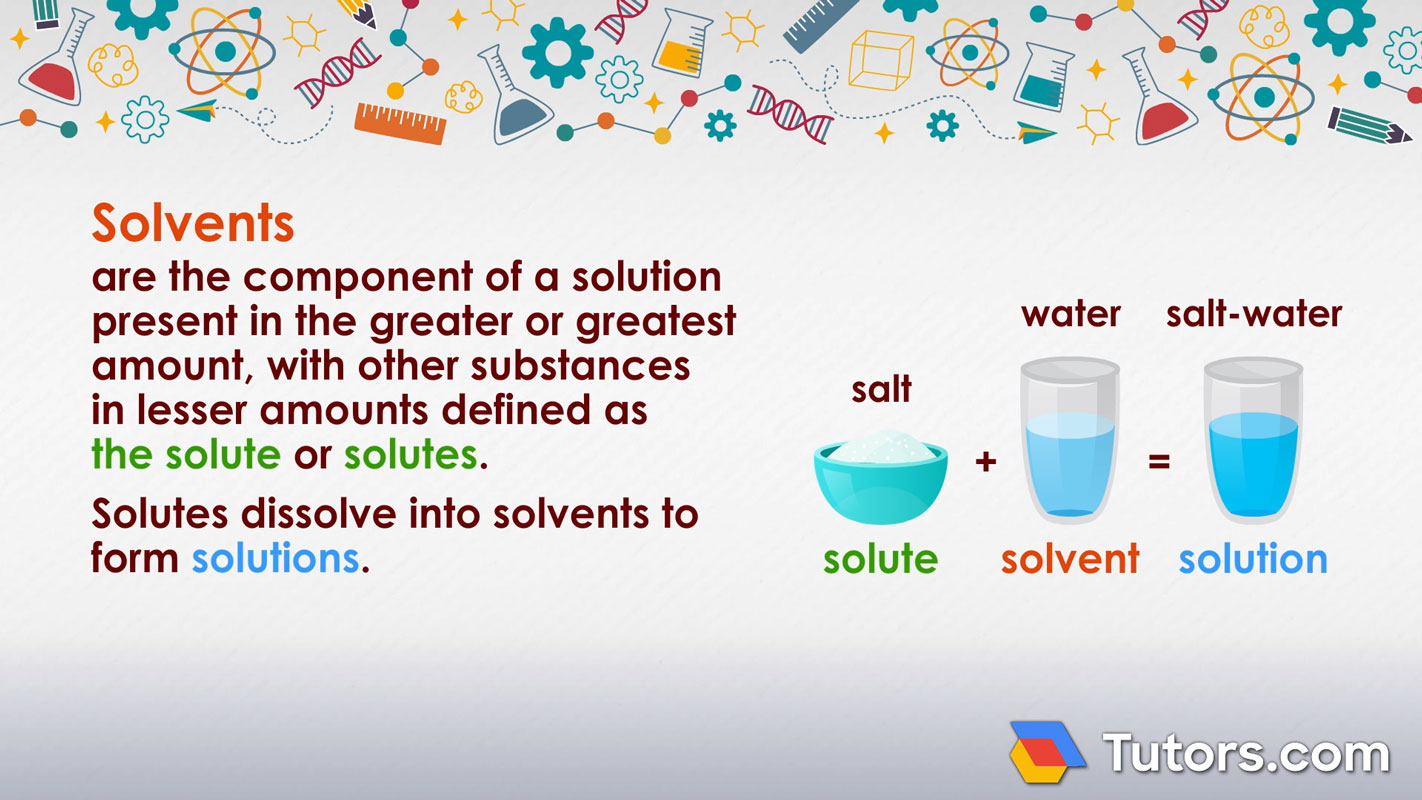
Solvents are the component of a solution present in the greater or greatest amount, with other substances in lesser amounts defined as the solute or solutes. Solutes dissolve into solvents to form solutions.
For example, in sugar water used to make lemonade, sugar water is the solution, sugar is the solute, and water is the solvent.
What is a polar solvent?
Water is a polar solvent, meaning it has an uneven distribution of electron density. For anything to dissolve in water, it must share water's polarity characteristics: the solute must be a polar compound or an ionic compound, like table salt.

Solute, solvent, and solution
A solute is any substance in a solution present in a lesser amount than the solvent. For example, a can of soda may have a list of ingredients of carbonated water, sugar, caramel coloring, phosphoric acid, and flavorings such as caffeine.
The carbonated water is the solvent, and every other listed ingredient is a solute. The ability for a solute to dissolve in a solvent is called solubility.
A solution is a homogeneous mixture, meaning it is a combination of substances so thoroughly mixed as to appear to be one substance. Every sample of a solution will be the same as every other sample.
Solutions can be solids, liquids, gases, or any combination of these, as with liquid and gas solutions used to make drinkable soda.
Solvent examples
Solutions are all around you. You are breathing a solution of gases every moment of your life. The gas present in the greatest amount in the air we breathe is nitrogen, , which makes up about 78% of our atmosphere. Therefore, nitrogen is the solvent in the air.
Everything else, including the oxygen we need to stay alive, is a solute. Air is a gas-gas solution.
Solutions can be solid-solid, such as steel. To make steel, factory workers use anywhere from 98% to 99% iron, , and only one to two percent carbon, . Iron is the overwhelming solvent in making steel.

To make variations of steel, such as stainless steel, manufacturers add chromium to steel. Then, the steel itself becomes the solvent, and the chromium is the solute.
Solvents and solutes can appear in any of the most common forms of matter in solutions, as solids, liquids, or gases. Solid solutes can dissolve in liquid solvents, such as salt in the water to make saltwater for gargling.
Common solvents
Here is a list of solvents, some you can find in your home:
Acetone
Alcohol (ethanol)
Water
Bleach
Methanol
Benzene
Chloroform
Paint thinners
The liquid soap you use to wash your hands several times a day is always made with water as the main ingredient, making water the solvent for all the various chemicals, colors, and fragrances added to make the soap appealing and effective.
Bleach, which is used to disinfect laundry, is the solid compound sodium hypochlorite, the solute dissolved in water, the solvent.
Ammonia, , is a strong cleaning agent, a base, and is often the main ingredient of window cleaners and disinfectants. Ammonia in any of these cleaners would be the solvent.
When you brew tea or coffee and add milk, honey, or sugar to your beverage, the tea or coffee becomes the solvent, and the milk, honey, or sugar is the solute.
Water is known as the “universal solvent” because many substances dissolve in water. The reason so many substances dissolve in water is due to the high polarity of the water molecule.

Solvents quiz
See if this all makes sense to you by answering these self-check questions. Then check your answers against ours below.
Identify the two parts of any solution.
In what phases of matter can solvents appear?
Please give an example of a liquid solvent with a gaseous solute.
Please give an example of a common household solution and its solvent.
What substance is known as the universal solvent?
Please work carefully and then check your ideas against these answers. If in doubt, reread this article and do more research on your own!
Two parts of any solution are the solvent and the solute.
Solvents can appear in every phase of matter in solutions, from solids like iron in the steel to liquids like water in sugar water to gases like nitrogen in the air we breathe.
An example of a liquid solvent with a gaseous solute is sugar water with carbon dioxide gas dissolved in it, a basic combination of all sweetened sodas.
An example of a common household solution and its solvent is bleach, which is water with sodium hypochlorite dissolved in it, or liquid soap, which is water with detergents dissolved in it.
The substance known as the universal solvent is pure water.