Chemical Change — Definition, Properties, Types & Examples
Chemical change definition
A chemical change is a reaction that alters the chemical composition of a pure substance. They occur when a pure substance becomes one or more different pure substances. There are several types of chemical changes.
Properties of chemical changes
Every chemical change involves a chemical reaction, which changes the chemical bonds of the original substance.
Chemical reactions create one new chemical substance or several new substances (the products) from one or more reactants.
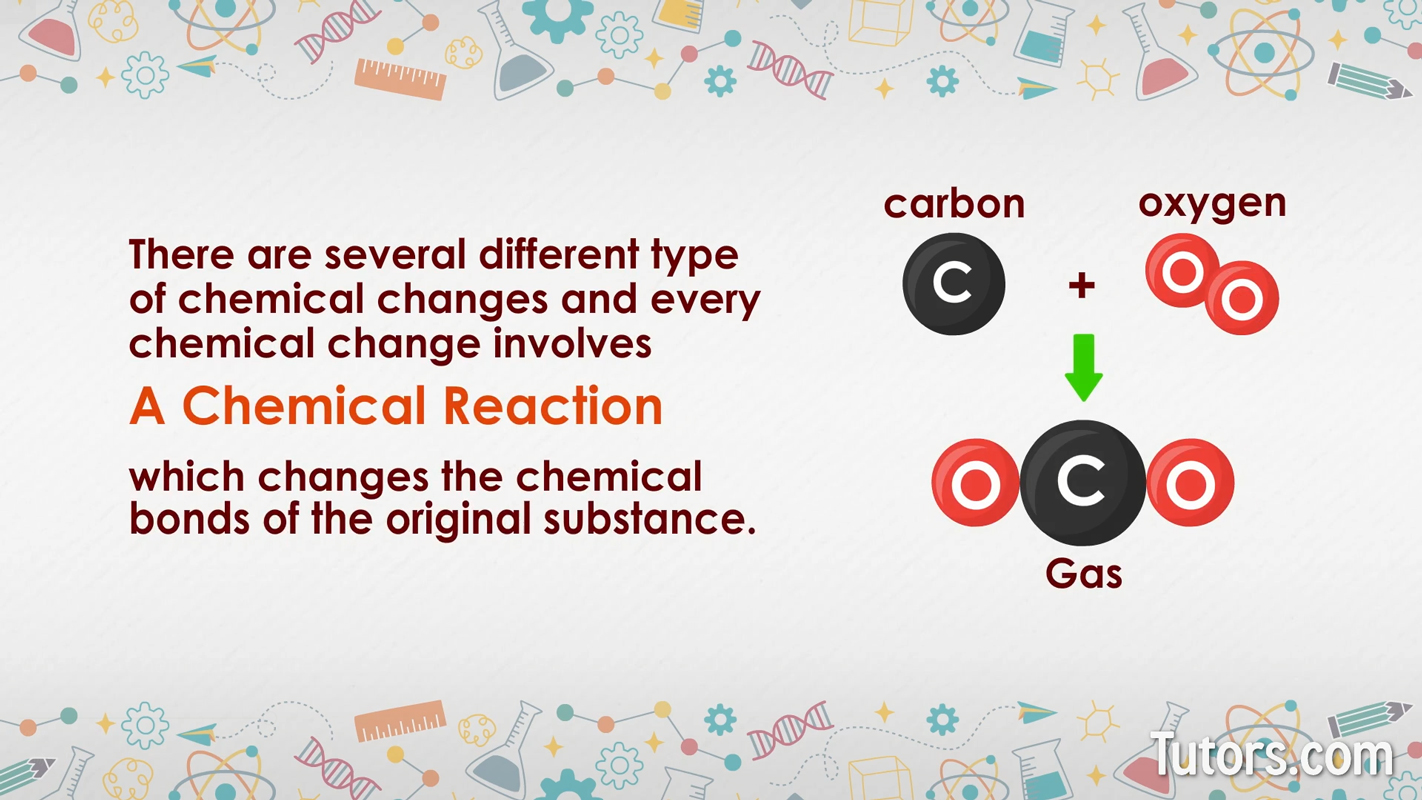
The products of a chemical reaction have different chemical properties and usually different physical properties than the reactants.
You can help yourself if you learn to recognize all the characteristics of chemical change.
Production of heat, light, or sound
Production of gas not present in the reactants
A permanent change in color
Production of a precipitate
Change in odor or taste
Change in density
Change in temperature

Observing only one reaction is not enough to know you have a chemical change and not a physical change.
Expect to see several characteristics from this list of seven signs of a chemical change.
Types of chemical changes
We see many types of chemical changes all around us and even within our bodies.
Here are eight types of chemical reactions:
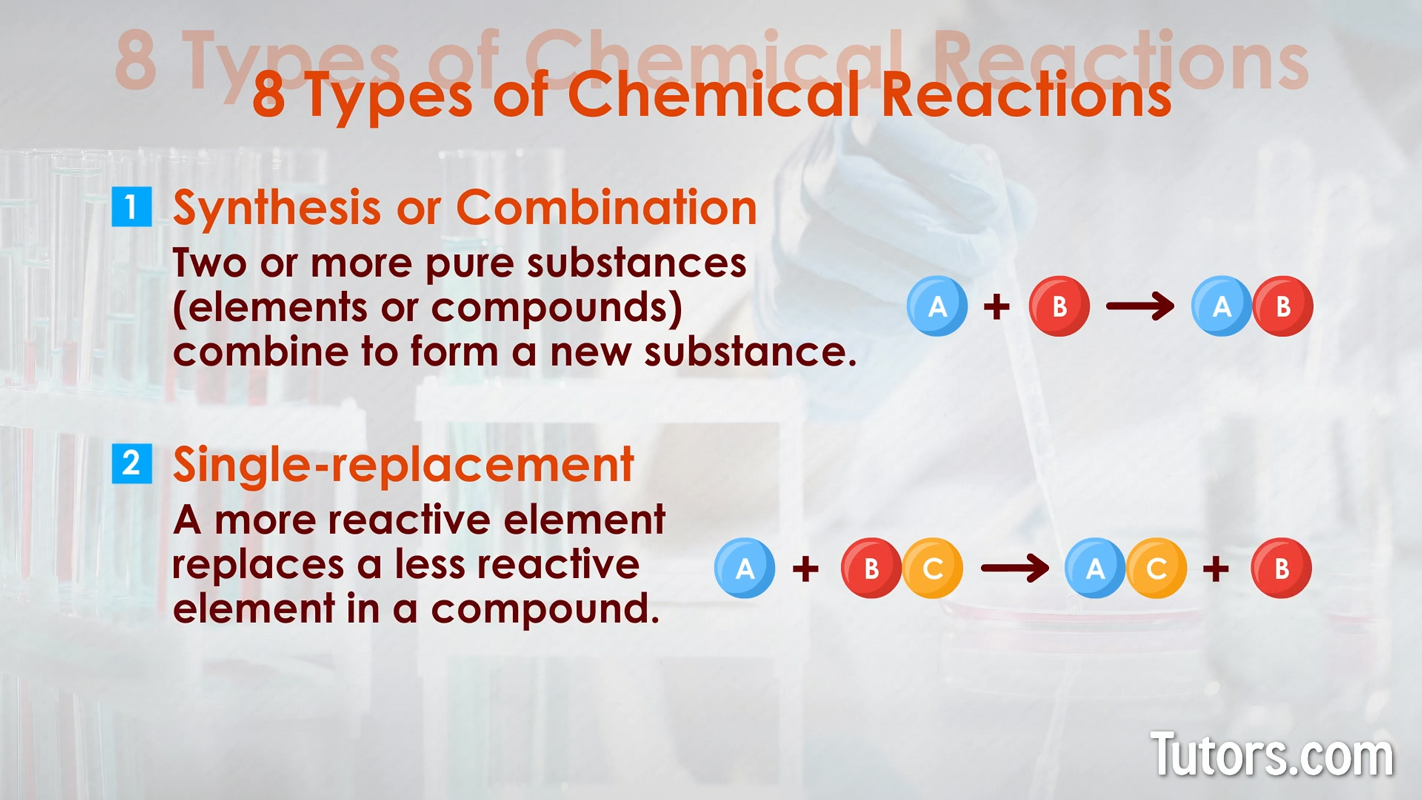
Synthesis or Combination – Two or more pure substances (elements or compounds) combine to form a new substance. The generic synthesis formula is:
Single-replacement – A more reactive metal replaces a less reactive metal, or a reactive nonmetal replaces a less reactive nonmetal in a compound. A single replacement generic formula is:
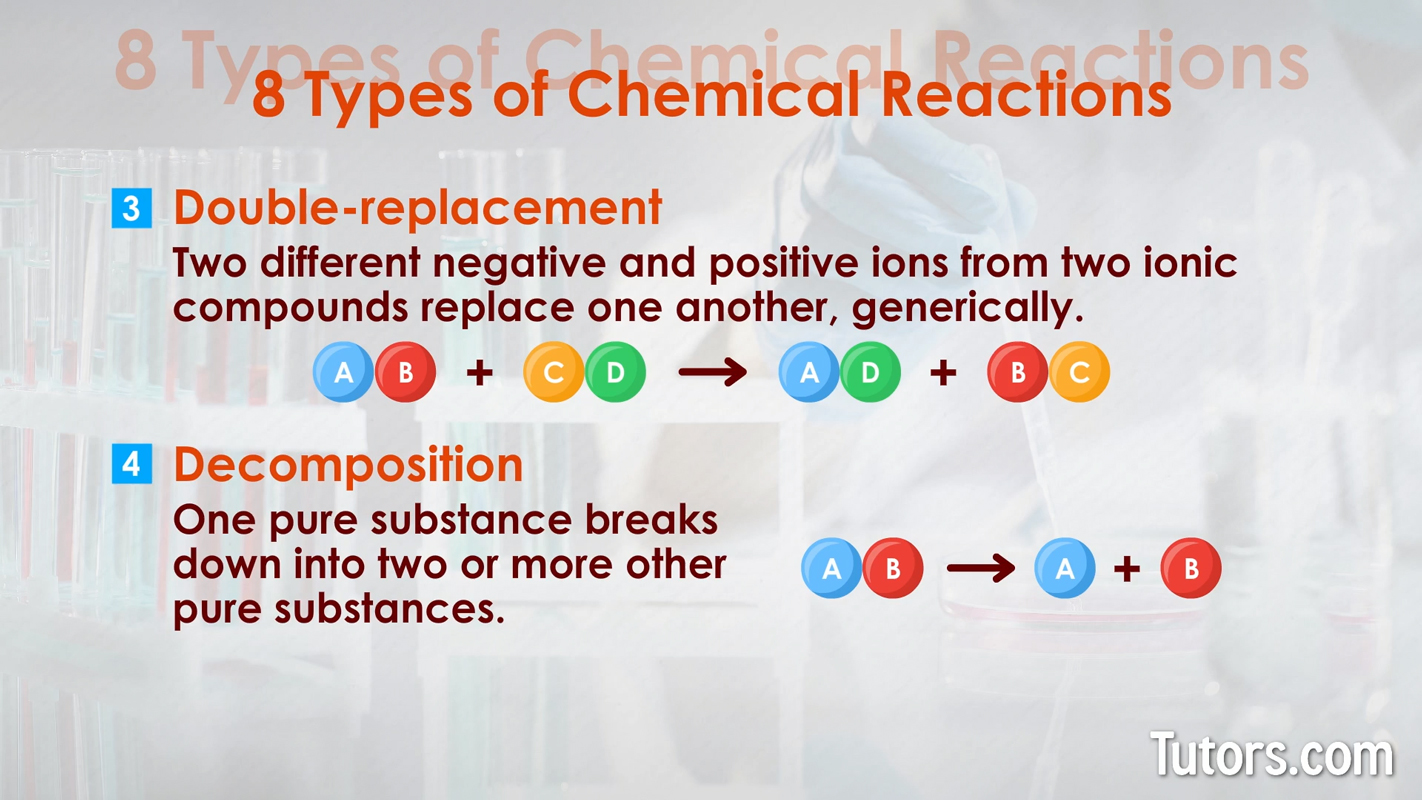
Types of chemical changes - synthesis and single replacement Double-replacement – Two different negative and positive ions from two ionic compounds replace one another. Generically, double replacement is shown as:
Decomposition – One pure substance breaks down into two or more other pure substances. The generic formula is:
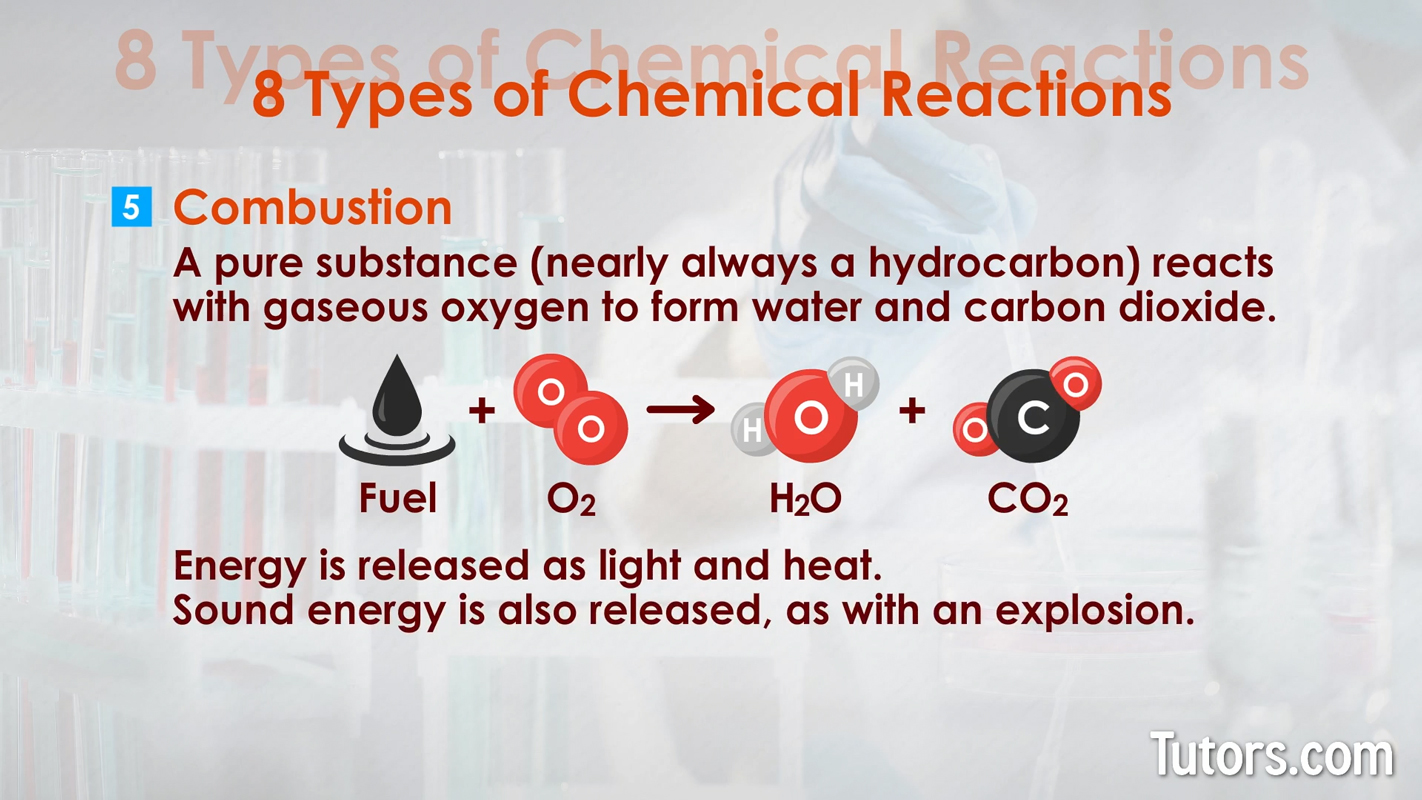
Types of chemical changes - double replacement and decomposition Combustion – A pure substance (nearly always a hydrocarbon) reacts with gaseous oxygen to form water and carbon dioxide. Energy is also released as light and heat. Sometimes, sound energy is also released, as with an explosion. The generic formula is:
Types of chemical changes - combustion Reduction/Oxidation (redox) – The general term for chemical changes that involve the transfer of electrons from one element to another is a redox reaction. Synthesis, single-replacement, and combustion reactions are all categorized as redox (reduction-oxidation) reactions. The generic formula is because compound or element is oxidized () while compound or element is reduced (). lost an electron, and gained an electron.
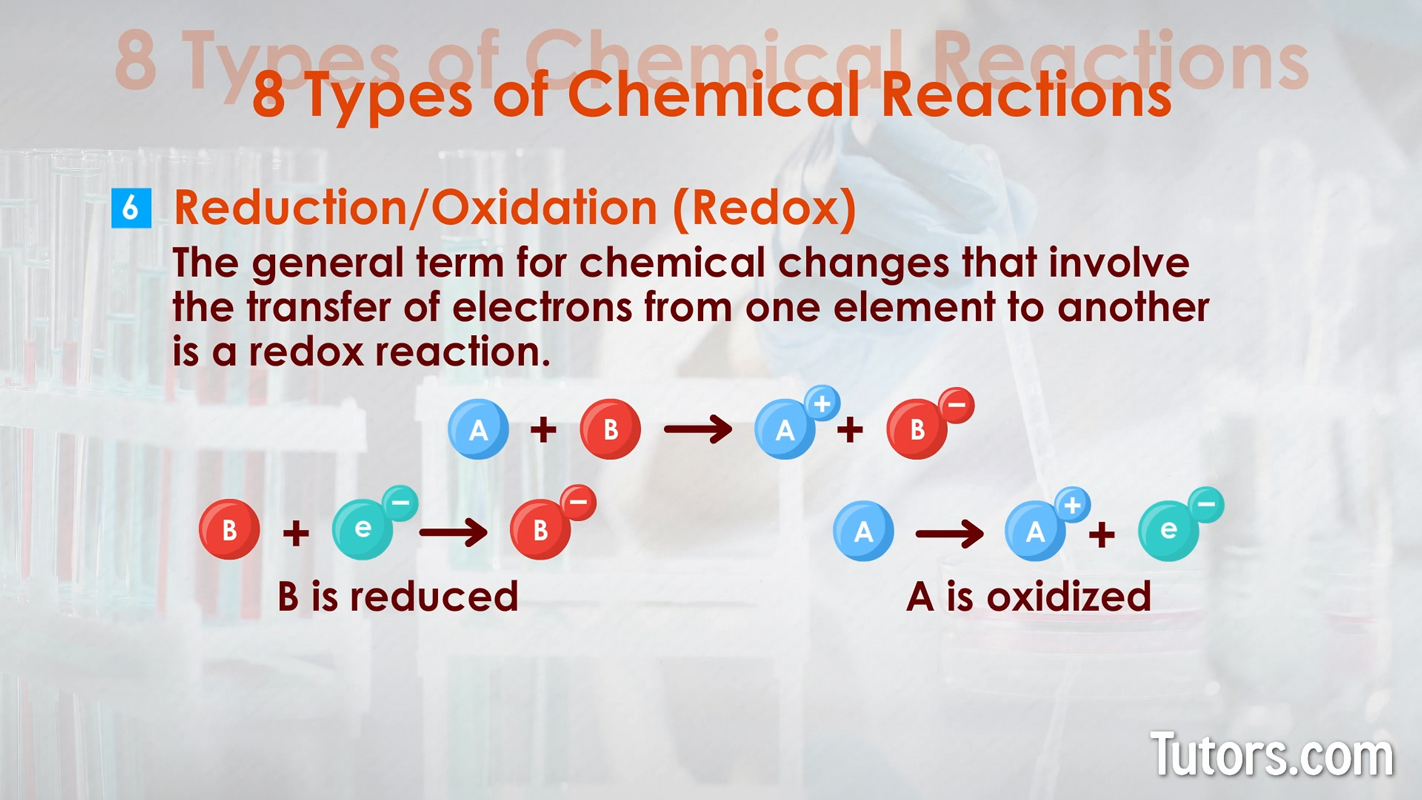
Types of chemical changes - reduction oxidation (redox) Precipitation – Forming an insoluble solid in a solution is a precipitation reaction. Cations and anions in the liquid solution are the reactants, with the precipitate as the product. Precipitates form from either single-replacement or double-replacement reactions.
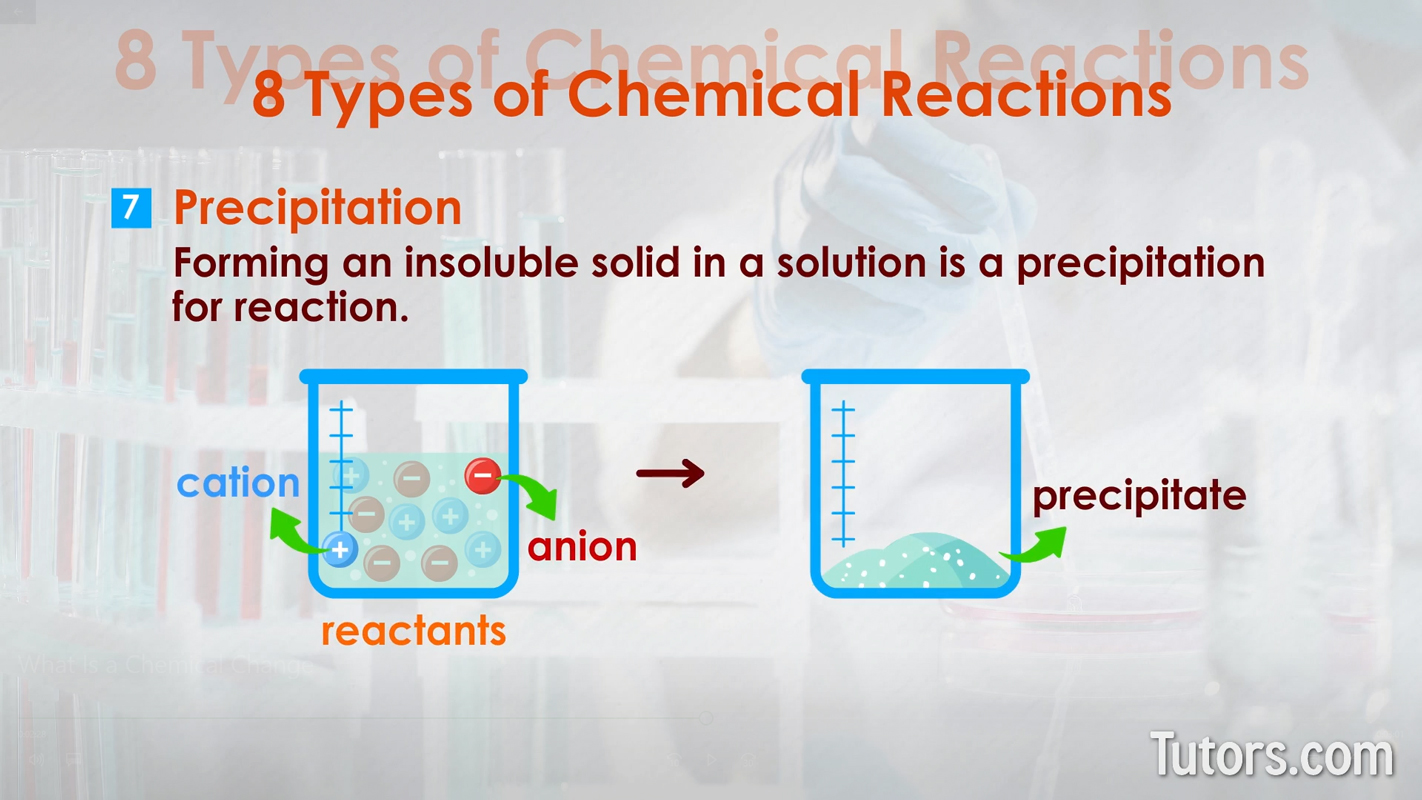
Types of chemical changes - Precipitation Neutralization – This type of double-replacement reaction between acids and bases neutralizes both the acid and base, producing water and salt.
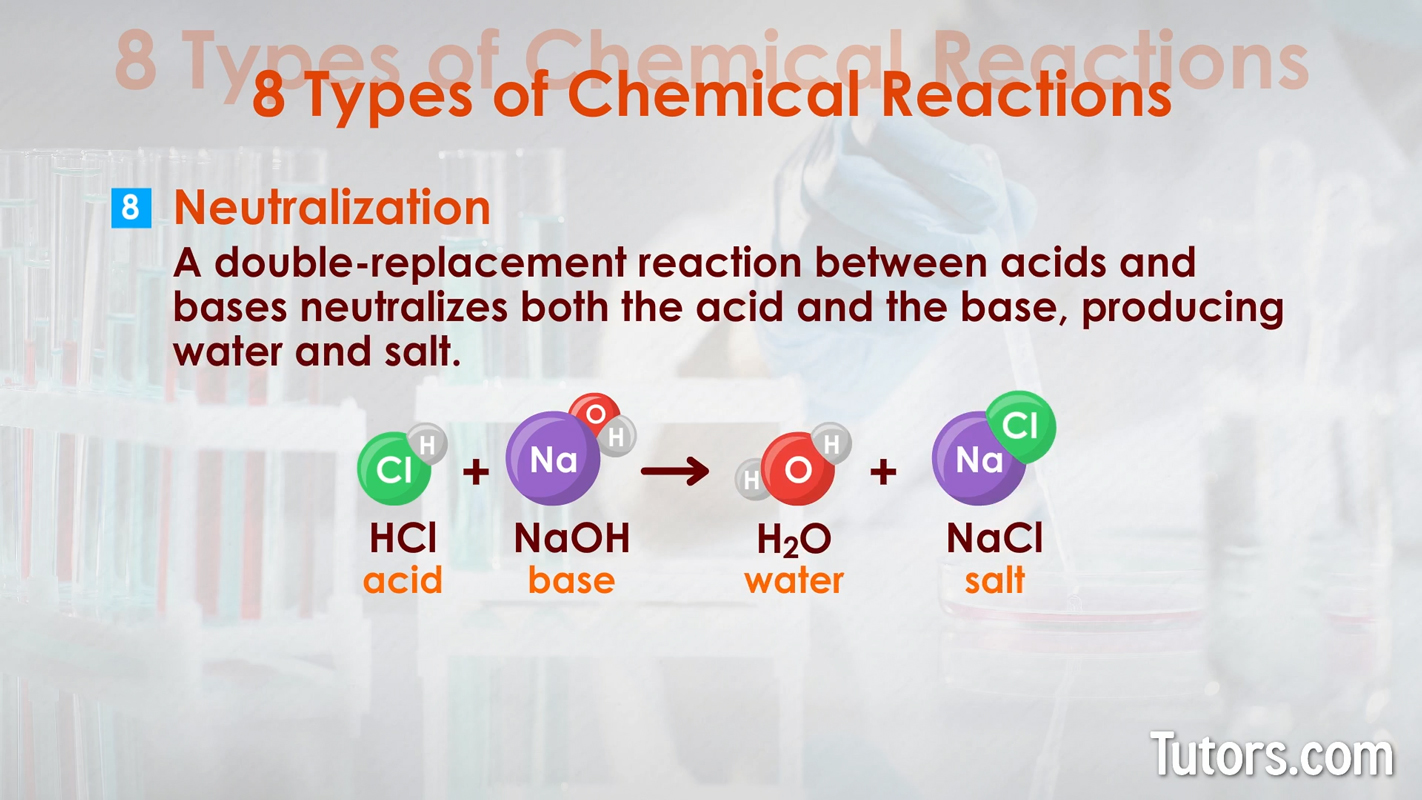
Types of chemical changes - neutralization
Chemical changes can occur quickly, as with combustion. Chemical changes can occur slowly, as with iron rusting into iron oxide or your body growing from a baby to an adult.
Chemical change examples
Imagine that you receive an unwelcome note on a scrap of paper from a classmate. What can you do? Burn it, cut it up, fold it into a paper airplane?
You can do a lot of physical and chemical things to a sheet of paper.
Burning the unwanted paper is an example of a chemical change.
You cannot get back the original paper because you destroyed the paper itself. No matter what you try, you cannot recover the paper and the writing.
For example, an explosion is a combustion reaction that will produce heat, light, sound, gas, a change in color, and a temperature change.
When oxygen () reacts with fuel, it chemically changes to carbon dioxide () and water () while releasing energy. That is a combustion reaction; it is a chemical change.
Chemical changes in biology are known as biochemical changes. Two examples of biochemical changes include fermentation and photosynthesis.
What does a chemical change produce?
The single most important result of a chemical change is the permanent transformation of the original substance. Chemically changing a substance produces a completely different substance - the matter changes the chemical makeup completely.
The products from the chemical change will also have almost no chemical properties or physical properties in common with the reactants. That piece of paper was cellulose; now, it is ash (carbon and some other compounds). The paper (the reactant) will never return.
Iron is magnetic, while iron sulfide that results from the synthesis of iron and sulfur is not magnetic. All chemical changes are one-way changes. Old substances transform into new substances.
Physical vs. chemical changes
Chemistry and physics are better understood when you know how to classify physical and chemical changes. If you alter the shape, size, or state of matter of some object but still have the object itself (in some form), you have performed a physical change.
Physical changes alter the shape, size, or state of matter of a substance. They do not alter chemical composition.
Chemical changes do alter chemical composition.

Changes in states of matter include four physical changes for the four states of matter (solids, liquids, gases, and plasmas).
Specific names for these changes apply to liquids and solids, solids and gases, gases and liquids, and gases and plasmas.
Here is a list of reactions that physically change the state of matter; each one has its opposite:
Freezing and Melting
Vaporizing and Condensing
Depositing and Ionizing
Recombining and Sublimating
With all physical changes, you can get back the original substance. Some physical changes are harder to reverse than others, but all physical changes can be “undone.”
Is ice melting a chemical change?
Melting an ice cube may show a change in temperature, but that is only one of eight signals, which is not enough to say that melting is a chemical change.
When ice (solid water) physically changes into liquid water, it is still water ().
Chemical change quiz
See how well you understand chemical changes by tackling these questions.
Is decomposition a chemical or physical change?
Name three types of chemical changes.
Name four characteristics of a chemical change.
What is the result of a chemical change?
Chemistry is challenging material, but please try your best before you peek at the answers below.
Decomposition is a chemical change in which one pure substance breaks down into two or more pure substances (elements or compounds).
You could have selected any three types of chemical changes from these: synthesis or combination, decomposition, single-replacement, double-replacement, combustion, neutralization, precipitation, or redox.
Did you name any four characteristics of a chemical change from the list of seven? Production of gas not present in the reactants; permanent color change; change in temperature; production of heat, light, or sound; change in odor or taste; production of a precipitate; change in density.
The result of a chemical change is the permanent loss of the reactants in the production of an entirely new substance. The resulting product almost always has chemical and physical properties, unlike those of the reactants.